Draw resonance structures for following compounds Show electron shift using curved arrow notation Draw the resonance structures for the following compounds. O Step 1 Your answer is incorrect First add curved arrows to show the resonance using the following patterns a pi bond between two atoms of differing electronegativity Modify the second structure given to draw the new resonance structure.
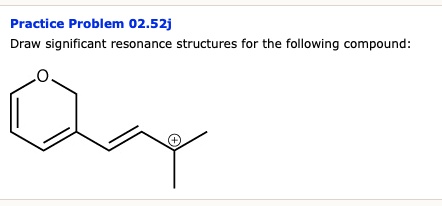
Solved Practice Problem 02 52j Draw Significant Resonance Structures For The Following Compound
Include lone pairs and charges in your structure.

. Draw significant resonance structures for the following compound. 1 Do not exceed the octet on 2nd-row elements. Be very careful to show where the curved arrow starts and where it ends O.
You can see first two structures have charges on atoms. Organic Chemistry 222 Fall 2016 Recitation Problem Set 2 Sep 13th 11 a Use resonance structures to help you identify all sites of low electron density d in the following compound. Draw all significant resonance structures for each of the following compounds.
Modify the second structure given to draw the new resonance structure. So lets go ahead and draw another residents structure. Therefore we can draw resonance structures for O 3 molecule as follows.
Move negative charge on oxygen. We can draw three resonance structures for SO 2 molecule. Transcribed image text.
An allylic lone pair. Two resonance forms are given below but they are incomplete. Draw all significant resonance structures for the following compound.
15 You can expect to have three more resonance structures for this anion. There are also several patterns for drawing resonance structures of radicals. Okay these are major resonance contributors for this structure.
What is the IUPAC name for the following compound. So we can go ahead and draw the residents arrows. 2 Do not break single bonds.
Draw the two remaining resonance structures in any order including nonbonding electrons and formal charges HaC Ha H3 Which structure is the. Resonance in chemistry could be a manner of describing the bonding in particular molecules or ions by merging many contributory structures or forms jointly called canonical structures or resonance structures within the theory of valence bonding into a hybrid resonance or hybrid structure. Remember to push electron pair step by step.
O First add curved arrow s to show the resonance using the following pattern. Two must-follow rules when drawing resonance structures. Draw all significant resonance structures for each of the following compounds.
N H N H N H a N N N b N N c C N O C N O d S O S O e f h O O i C N C N C N C N j k O OH l H. Complete the given structures by adding nonbonding electrons and formal charges. Include lone pairs and charges in your structure.
Rules for drawing resonance structures. Dont draw resonance structure within the aromatic ring. As a result the C-H bond is almost completely broken in the transition state and the carbon atom has significant radical character Figure 1014.
We can obtain the following resonance structures by following the same steps as mentioned above. Learn this topic by watching Resonance Structures-1 Concept Videos All Organic Chemistry Practice Problems Resonance Structures-1 Practice Problems. So we can understand structure three is more stable than other two structure.
Draw significant resonance structures for the following compound. Four resonance structures of the following cation are possible. Draw resonance structures for the following using curved arrows.
Students also viewed these Sciences questions Consider the structure of. Sulfur dioxide SO 2 molecule resonance structures. The different resonance structures of the carbonate ion CO 3 2- are illustrated.
Draw significant resonance structures for the following compound. The second-row elements C N O F can only handle up to. Draw all significant resonance structures for each of the following compounds.
This way these two are resonating with each other to form this compound. It gives the following structure and has a multiple bonds and an adjacent atom with one lone pair of electrons. Nitrogen dioxide NO 2 molecule.
We go ahead down to the next structure we can do the same thing with our character rose. But in third structure there are no charges on atoms.
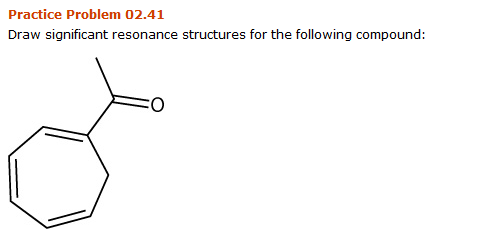
Solved Practice Problem 02 41 Draw Significant Resonance Chegg Com

Solved Draw Significant Resonance Structures For The Chegg Com
Solved Draw All Significant Resonance Structures Of The Following Compounds And Label The Most Significant Contributor S Draw In Any Missing Lon Course Hero
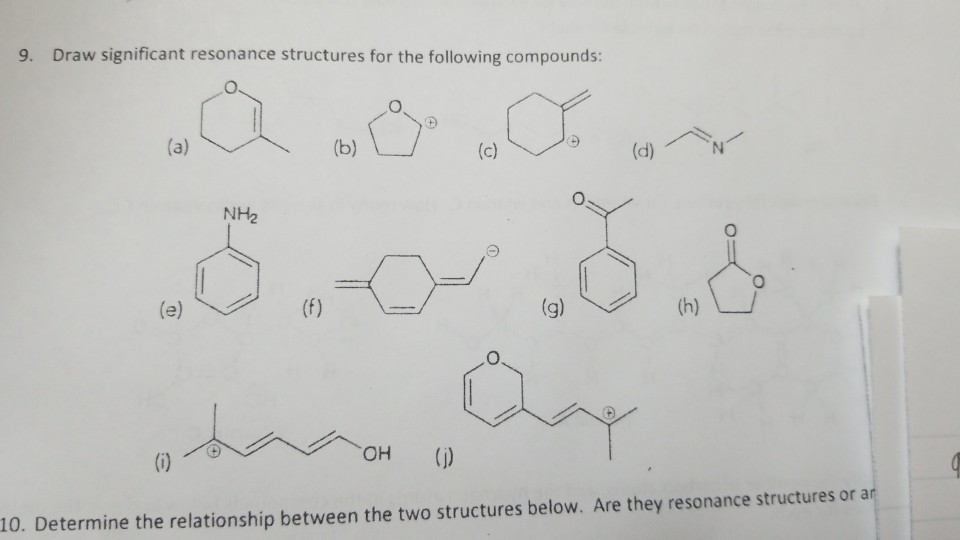
Solved 9 Draw Significant Resonance Structures For The Chegg Com

Draw Significant Resonance Structures For The Following Compound Which Of This Is Are Most Significant Resonance Structures Study Com

Solved 2 41 Draw Significant Resonance Structures For The Chegg Com

Draw Significant Resonance Structures For The Following Compound Which Of This Is Are Most Significant Resonance Structures Study Com

Solved Practice Problem 02 41 Draw Significant Resonance Chegg Com
0 komentar
Posting Komentar